Name of Journal: World Journal of Gastroenterology
Manuscript NO: 47278
Manuscript Type: OPINION REVIEW
Issues and controversies in esophageal inlet patch
Ciocalteu A et al. Esophageal inlet patch
Adriana Ciocalteu, Petrica Popa, Mircea Ionescu, Dan Ionut Gheonea
Telephone: +40-74-8374019
Fax: +40-251-310287
Peer-review started: March 12, 2019
First decision: June 10, 2019
Revised: June 24, 2019
Accepted: July 5, 2019
Article in press:
Published online:
The proximal esophagus is rarely examined, and its inspection is often inadequate. Optical chromoendoscopy techniques such as narrow band imaging improve the detection rate of inlet patches in the proximal esophagus, a region in which their prevalence is likely underestimated. Various studies have reported correlations between these esophageal marks with different issues such as Barrett’s esophagus, but these findings remain controversial. Conflicting reports complicate the process of interpreting the clinical features of esophageal inlet patches and underestimate their importance. Unfortunately, the limited clinical data and statistical analyses make reaching any conclusions difficult. It is hypothesized that inlet patches are correlated with various esophageal and extraesophageal symptoms, diagnoses and the personalized therapeutic management of patients with inlet patches as well as the differential diagnosis for premalignant lesions or early cancers. Due to its potential underdiagnosis, there are no consensus guidelines for the management and follow up of inlet patches. This review focuses on questions that were raised from published literature on esophageal inlet patches in adults.
Another study speculated that mucus secretion rather than acid production could be the cause of symptoms in patients with globus sensations that were unresponsive to PPI therapy[20]. In this small population of patients, histopathologic examinations revealed only the presence of cardiac mucosa.
In 2013, Chong et al[9] could only find 43 cases of esophageal cancers in the literature that presented concomitantly with heterotopic gastric mucosa since 1950 when Carrie et al[53] reported the first case.
Furthermore, Sahin et al found no cases of adenocarcinoma or dysplasia and detected additional intestinal metaplasia in only five of 123 IP cases[30].
The lack of studies with long-term follow-ups for IP might be a source of this bias. Other authors such as Peitz et al[11] have also considered that the prevalence of IPs is underestimated, making a correlation with advanced cervical esophageal cancer difficult. Due to the rare incidence of preneoplasia reported for IP, the authors do not support the routine biopsy to determine its histopathology, but rather targeted biopsies should be considered whenever any irregularities within the area are observed. In addition to this opinion, there are technical difficulties in typically occurring region located in upper esophagus (contractions of the upper esophageal sphincter or low tolerance of unsedated patients), so the routine biopsy should be limited to atypical locations of the IPs (e.g., distal or middle part of the esophagus) or for atypical appearances (e.g., polypoid types). For symptomatic patients with usually located IPs, and when confirmation is not possible by biopsy, a virtual chromoendoscopy with selected follow up cases could be helpful.
Confocal laser endomicroscopy could avoid both doctor and patient anticipatory anxiety related to a proper diagnosis. Unfortunately, this technique’s feasibility for routine use is impaired by increased costs and limited access.
Detection of IP-like lesions and subsequent confirmation by histology would help to avoid confusing incipient cancers with heterotopic mucosa. IPs present with a reddish or salmon-rose colored focal area on standard endoscopy and as a homogeneous dark brown lesion that is distinctly separated from the light green squamous epithelium in the NBI mode[54]. NBI systems can be very helpful to identify brownish areas with brown dots and branching vessels in the cervical esophagus as potential superficial esophageal cancers. Therefore, the combined application of magnification and NBI can help to inform and direct the diagnostic management and early detection of esophageal neoplasia[55]. Magnifying endoscopy with the NBI system is superior to conventional white-light endoscopy for the detection of early cancers and helps to resolve the microvascular patterns of the superficial esophageal mucosa[56,57]. Ideally, a future implementation of an automatic detection system for early neoplasia similar to the automated computer algorithm developed for incipient neoplasia in BE that proposed by Fons van der Sommen et al[58] could be implemented (Figure 4).
Whether the IP increases the risk of esophageal carcinoma remains controversial. Acid secretion was also a suspected cause of malignant transformation[59], but there is a discrepancy between the symptomatic acid-related IP prevalence and the rarely reported cases of malignization. There are likely other simultaneous risk factors that are involved. However, considering that cancers in IPs are typically reported as isolated cases[60–62], the focus should remain on being able to accurately differentiate between harmless IPs and superficial malignancies. As white-light endoscopies may not reveal the abnormal features of early neoplasias, the routine use of virtual chromoendoscopy in the esophagus is justifiable. Underreporting the incidence of IPs by endoscopy must be avoided and future studies should be performed to reach more pertinent conclusions.
Symptoms and their response to treatment may depend on a range of factors such as the type of heterotopic mucosa, H. pylori colonization and extraesophageal IP factors, but further studies are necessary to reach firm conclusions.
Going forward, the focus should remain on reassuring the patient and the routine use of virtual chromoendoscopy in the proximal region of the esophagus to direct the appropriate collection of biopsies from the IP-like mucosa. Another concern is whether surveillance is necessary after identifying an IP. Currently, and potentially due to its place as an underdiagnosed entity, there are no consensus guidelines for the management and follow up of IPs.
As there was no demonstrated association between the histopathology and clinical symptoms of the IP[59], symptomatic patients should be treated and considered for endoscopic reevaluation when other complications of the heterotopic gastric mucosa are suspected[30]. In selected cases, such as patients who are at a high risk of neoplasia or patients who are symptomatic, elderly, or smokers[64], the IP should be systematically evaluated and meticulously described with an endoscopic diagnosis and the patient should be considered for surveillance. Von Rahnen’s classification used in conjunction with the NBI description could be included in the endoscopic report to improve awareness of any potential evolution of the lesion during the next evaluation.
When a follow up is scheduled, the patient can be offered sedation for the second evaluation to provide a better examination or more accurate biopsy sampling. A minimum of two biopsies should be performed depending of the size of the inlet mucosa. An uncomplicated IP suggests a similar therapeutic attitude to functional dyspepsia or to nonerosive reflux disease. A differential diagnosis is required to determine which patients will benefit from alternative strategies. Since independent acid secretion episodes are a likely symptomatic cause, PPI and/or antacids paired with psychological reassurance should be the initial treatment option for symptomatic patients. If patient anxiety is observed, a low dose anxiolytic can be included. Prokinetic agents may also help any abnormal local motility. Previous studies reported a significant reduction in the number of symptoms from patients on acid suppression therapy such as a PPI treatment[65–67].
The duration of PPI administration is not clearly defined, but we have determined that therapy sessions such as “step-down” or “step-up” for 4-8 weeks, which are similar to GERD treatment, followed by on demand PPI can be effectively applied. If there are recurrences despite a high dose of PPI, adding H2 receptor antagonists in the evening to the PPI in the morning can prevent the breakthrough of nocturnal acid secretion. Of course, future studies and more data are required to prove the efficacy of this strategy[68–71]. However, continuous and long-term use of both PPI and H2 blockers should be discouraged to avoid developing resistances, rebound acid reflux and adverse effects. Long-term use of PPI also raises the question if it could influence the development of the heterotopic mucosa of the intestinal metaplasia or atrophy. Interestingly, one study reported that lesions were reduced in size after a course of PPI 20 mg, twice daily[72].
Similar gastric histological changes (inflammation, metaplasia, atrophy dysplasia and even adenocarcinoma of the IP with H. pylori colonization) have been reported[9]. Although there are insufficient data to recommend testing and eradicating H. pylori infections among patients with laryngopharyngeal reflux[73], we suggest that the endoscopist should consider searching for cervical IPs. Then, a rapid urease test from the IP can be considered to determine the presence of H. pylori in patients with an unexplained persistent globus sensation or a dyspepsia despite the PPI treatment and without H. pylori-positive gastritis, or to decide to pursue further treatment in patients with persistent dyspepsia after previous gastric H. pylori eradication. In both the stomach and ectopic mucosa with H. pylori infections, eradication issues could also be taken into consideration such as different antibiotic susceptibilities and resistances.
In symptomatic patients with the typical aspects of IPs who are unresponsive to PPI, endoscopic therapy, such as argon plasma coagulation or radiofrequency ablation, have also been reported to be safe and effective[34,74]. However, in our opinion, the clinical management should be kept as noninvasive as possible so long as there are no unfavorable outcomes, complications or any suspicion of neoplasia. Endoscopic treatment is not only technically challenging due to the typical position of the IP in the proximal esophagus, but may also only be available in dedicated centers.
Strictures and webs can be managed by serial dilatation and biopsied to rule out malignancy[7,33]. A high-dose PPI paired with endoscopic thermal coagulation led to long-term amelioration of dysphagia in one case of IP with stricture and even to the recovery of the mucosa with normal squamous epithelium[75]. Endoscopic mucosectomy (EMR), argon plasma coagulation (APC) or surgical resection has also been used to successfully treat IP dysplasia or incipient neoplasia[7,15,74,76,77], although the routine use of these strategies in this context has not been studied.
Other issues such as elevated surfaces[78] or the size of the IP should be taken into consideration before deciding which strategy is most appropriate. For instance, experts generally did not include patients with large IPs in the previously conducted interventional APC trials to exclude the possibility of stricture formation[18,79–81]. Furthermore, large areas of resected tissue and multiple lesions were independent predictors of stricture formation[82] (Figure 5).
In contrast, Kristo et al[78] recently reported an 80% rate of complete macroscopic and histologic eradication after 2 sessions of radiofrequency ablation with improvements in globus sensation and quality of life without any major adverse events or stricture formation after an approximate 2-year follow-up. The involvement of the esophageal heterotopic mucosa in esophageal pathology may eventually become as popular as BE, which will promote novel technologies such as hybrid-APC that could improve the therapeutic intervention for selected cases of large IPs in the future[83,84]. Confocal laser endomicroscopy could enable in vivo examinations of histology for flat lesions in the cervical esophagus in order to avoid a number of unnecessary biopsies and to direct any further EMR or endoscopic submucosal dissections[85].
REFERENCES
1 Raine CH. Ectopic gastric mucosa in the upper esophagus as a cause of dysphagia. Ann Otol Rhinol Laryngol 1983; 92: 65-66 [PMID: 6824282 DOI: 10.1177/000348948309200115]
2 Truong LD, Stroehlein JR, McKechnie JC. Gastric heterotopia of the proximal esophagus: a report of four cases detected by endoscopy and review of literature. Am J Gastroenterol 1986; 81: 1162-1166 [PMID: 3788924]
3 Akbayir N, Alkim C, Erdem L, Sökmen HM, Sungun A, Başak T, Turgut S, Mungan Z. Heterotopic gastric mucosa in the cervical esophagus (inlet patch): endoscopic prevalence, histological and clinical characteristics. J Gastroenterol Hepatol 2004; 19: 891-896 [PMID: 15242492 DOI: 10.1111/j.1440-1746.2004.03474.x]
4 Borhan-Manesh F, Farnum JB. Incidence of heterotopic gastric mucosa in the upper oesophagus. Gut 1991; 32: 968-972 [PMID: 1916499 DOI: 10.1136/gut.32.9.968]
5 Tang P, McKinley MJ, Sporrer M, Kahn E. Inlet patch: prevalence, histologic type, and association with esophagitis, Barrett esophagus, and antritis. Arch Pathol Lab Med 2004; 128: 444-447 [PMID: 15043461 DOI: 10.1043/1543-2165(2004)128<444:IPPHTA>2.0.CO;2]
6 Avidan B, Sonnenberg A, Chejfec G, Schnell TG, Sontag SJ. Is there a link between cervical inlet patch and Barrett’s esophagus? Gastrointest Endosc 2001; 53: 717-721 [PMID: 11375577 DOI: 10.1067/mge.2001.114782]
7 von Rahden BH, Stein HJ, Becker K, Liebermann-Meffert D, Siewert JR. Heterotopic gastric mucosa of the esophagus: literature-review and proposal of a clinicopathologic classification. Am J Gastroenterol 2004; 99: 543-551 [PMID: 15056100 DOI: 10.1111/j.1572-0241.2004.04082.x]
8 Alagozlu H, Simsek Z, Unal S, Cindoruk M, Dumlu S, Dursun A. Is there an association between Helicobacter pylori in the inlet patch and globus sensation? World J Gastroenterol 2010; 16: 42-47 [PMID: 20039447 DOI: 10.3748/wjg.v16.i1.42]
9 Chong VH. Clinical significance of heterotopic gastric mucosal patch of the proximal esophagus. World J Gastroenterol 2013; 19: 331-338 [PMID: 23372354 DOI: 10.3748/wjg.v19.i3.331]
10 Jabbari M, Goresky CA, Lough J, Yaffe C, Daly D, Côté C. The inlet patch: heterotopic gastric mucosa in the upper esophagus. Gastroenterology 1985; 89: 352-356 [PMID: 4007426 DOI: 10.1016/0016-5085(85)90336-1]
11 Peitz U, Vieth M, Evert M, Arand J, Roessner A, Malfertheiner P. The prevalence of gastric heterotopia of the proximal esophagus is underestimated, but preneoplasia is rare – correlation with Barrett’s esophagus. BMC Gastroenterol 2017; 17: 87 [PMID: 28701149 DOI: 10.1186/s12876-017-0644-3]
12 Maconi G, Pace F, Vago L, Carsana L, Bargiggia S, Bianchi Porro G. Prevalence and clinical features of heterotopic gastric mucosa in the upper oesophagus (inlet patch). Eur J Gastroenterol Hepatol 2000; 12: 745-749 [PMID: 10929900 DOI: 10.1097/00042737-200012070-00005]
13 Januszewicz W, Pietrzak A, Lenarcik M, Mróz A, Reguła J. Long esophageal inlet patch as a rare cause of laryngopharyngeal symptoms. Endoscopy 2018; 50: E61-E62 [PMID: 29245163 DOI: 10.1055/s-0043-123823]
14 Orosey M, Amin M, Cappell MS. A 14-Year Study of 398 Esophageal Adenocarcinomas Diagnosed Among 156,256 EGDs Performed at Two Large Hospitals: An Inlet Patch Is Proposed as a Significant Risk Factor for Proximal Esophageal Adenocarcinoma. Dig Dis Sci 2018; 63: 452-465 [PMID: 29249048 DOI: 10.1007/s10620-017-4878-2]
15 Jacobs E, Dehou MF. Heterotopic gastric mucosa in the upper esophagus: a prospective study of 33 cases and review of literature. Endoscopy 1997; 29: 710-715 [PMID: 9427488 DOI: 10.1055/s-2007-1004294]
16 Neumann WL, Luján GM, Genta RM. Gastric heterotopia in the proximal oesophagus (“inlet patch”): Association with adenocarcinomas arising in Barrett mucosa. Dig Liver Dis2012; 44: 292-296 [PMID: 22222950 DOI: 10.1016/j.dld.2011.11.008]
17 Chong VH, Jalihal A. Heterotopic gastric mucosal patch of the esophagus is associated with higher prevalence of laryngopharyngeal reflux symptoms. Eur Arch Otorhinolaryngol2010; 267: 1793-1799 [PMID: 20437050 DOI: 10.1007/s00405-010-1259-2]
18 Bajbouj M, Becker V, Eckel F, Miehlke S, Pech O, Prinz C, Schmid RM, Meining A. Argon plasma coagulation of cervical heterotopic gastric mucosa as an alternative treatment for globus sensations. Gastroenterology 2009; 137: 440-444 [PMID: 19410576 DOI: 10.1053/j.gastro.2009.04.053]
19 Korkut E, Bektaş M, Alkan M, Ustün Y, Meco C, Ozden A, Soykan I. Esophageal motility and 24-h pH profiles of patients with heterotopic gastric mucosa in the cervical esophagus. Eur J Intern Med 2010; 21: 21-24 [PMID: 20122608 DOI: 10.1016/j.ejim.2009.10.009]
20 Weickert U, Wolf A, Schröder C, Autschbach F, Vollmer H. Frequency, histopathological findings, and clinical significance of cervical heterotopic gastric mucosa (gastric inlet patch): a prospective study in 300 patients. Dis Esophagus 2011; 24: 63-68 [PMID: 20626446 DOI: 10.1111/j.1442-2050.2010.01091.x]
21 López-Colombo A, Jiménez-Toxqui M, Gogeascoechea-Guillén PD, Meléndez-Mena D, Morales-Hernández ER, Montiel-Jarquín ÁJ, Amaro-Balderas E. Prevalence of esophageal inlet patch and clinical characteristics of the patients. Rev Gastroenterol Mex 2018; : [PMID: 30318401]
22 Takeji H, Ueno J, Nishitani H. Ectopic gastric mucosa in the upper esophagus: prevalence and radiologic findings. AJR Am J Roentgenol 1995; 164: 901-904 [PMID: 7726045 DOI: 10.2214/ajr.164.4.7726045]
23 Hori K, Kim Y, Sakurai J, Watari J, Tomita T, Oshima T, Kondo C, Matsumoto T, Miwa H. Non-erosive reflux disease rather than cervical inlet patch involves globus. J Gastroenterol 2010; 45: 1138-1145 [PMID: 20582442 DOI: 10.1007/s00535-010-0275-8]
24 Lauwers GY, Mino M, Ban S, Forcione D, Eatherton DE, Shimizu M, Sevestre H. Cytokeratins 7 and 20 and mucin core protein expression in esophageal cervical inlet patch. Am J Surg Pathol 2005; 29: 437-442 [PMID: 15767795 DOI: 10.1097/01.pas.0000155155.46434.da]
25 Feurle GE, Helmstaedter V, Buehring A, Bettendorf U, Eckardt VF. Distinct immunohistochemical findings in columnar epithelium of esophageal inlet patch and of Barrett’s esophagus. Dig Dis Sci 1990; 35: 86-92 [PMID: 2295298 DOI: 10.1007/BF01537228]
26 Wlaź J, Mądro A, Kaźmierak W, Celiński K, Słomka M. Pancreatic and gastric heterotopy in the gastrointestinal tract. Postepy Hig Med Dosw (Online) 2014; 68: 1069-1075 [PMID: 25228515 DOI: 10.5604/17322693.1119720]
27 Polat FR, Polat S. The effect of Helicobacter pylori on gastroesophageal reflux disease. JSLS 2012; 16: 260-263 [PMID: 23477175 DOI: 10.4293/108680812X13427982376860]
28 Ghoshal UC, Chourasia D. Gastroesophageal Reflux Disease and Helicobacter pylori: What May Be the Relationship? J Neurogastroenterol Motil 2010; 16: 243-250 [PMID: 20680162 DOI: 10.5056/jnm.2010.16.3.243]
29 Wüppenhorst N, Viebahn B, Theile A, Radü HJ, Kist M. Culture and successful eradication of Helicobacter pylori from heterotopic gastric mucosa. Z Gastroenterol 2012; 50: 677-679 [PMID: 22760679 DOI: 10.1055/s-0031-1299382]
30 Sahin G, Adas G, Koc B, Akcakaya A, Dogan Y, Goksel S, Yalcin O. Is cervical inlet patch important clinical problem? Int J Biomed Sci 2014; 10: 129-135 [PMID: 25018682]
31 Gutierrez O, Akamatsu T, Cardona H, Graham DY, El-Zimaity HM. Helicobacter pylori and hetertopic gastric mucosa in the upper esophagus (the inlet patch). Am J Gastroenterol 2003; 98: 1266-1270 [PMID: 12818267 DOI: 10.1111/j.1572-0241.2003.07488.x]
32 Akbayir N, Sökmen HM, Caliş AB, Bölükbaş C, Erdem L, Alkim C, Sakiz D, Mungan Z. Heterotopic gastric mucosa in the cervical esophagus: could this play a role in the pathogenesis of laryngopharyngeal reflux in a subgroup of patients with posterior laryngitis? Scand J Gastroenterol 2005; 40: 1149-1156 [PMID: 16265772]
33 Behrens C, Yen PP. Esophageal inlet patch. Radiol Res Pract 2011; 2011: 460890 [PMID: 22091379 DOI: 10.1155/2011/460890]
34 Shimamura Y, Winer S, Marcon N. A Giant Circumferential Inlet Patch With Acid Secretion Causing Stricture. Clin Gastroenterol Hepatol 2017; 15: A22-A23 [PMID: 27729241 DOI: 10.1016/j.cgh.2016.10.004]
35 Lin T, Linn S, Ona MA, Duddempudi S. Helicobacter pylori-positive inlet patch without concurrent Helicobacter pylori gastritis: case report of a patient with sleeve gastrectomy. Ann Gastroenterol 2017; 30: 251 [PMID: 28243049 DOI: 10.20524/aog.2016.0102]
36 Latos W, Sieroń-Stołtny K, Kawczyk-Krupka A, Operchalski T, Cieślar G, Kwiatek S, Bugaj AM, Sieroń A. Clinical evaluation of twenty cases of heterotopic gastric mucosa of upper esophagus during five-year observation, using gastroscopy in combination with histopathological and microbiological analysis of biopsies. Contemp Oncol (Pozn) 2013; 17: 171-175 [PMID: 23788986 DOI: 10.5114/wo.2013.34376]
37 Katsanos KH, Christodoulou DK, Kamina S, Maria K, Lambri E, Theodorou S, Tsampoulas K, Vasiliki M, Tsianos EV. Diagnosis and endoscopic treatment of esophago-bronchial fistula due to gastric heterotopy. World J Gastrointest Endosc 2010; 2: 138-142 [PMID: 21160729 DOI: 10.4253/wjge.v2.i4.138]
38 Ainley EJ. High oesophageal web formation in association with heterotopic gastric mucosa (the gastric inlet patch): a small case series. Frontline Gastroenterol 2011; 2: 117-123 [PMID: 28839593 DOI: 10.1136/fg.2010.002311]
39 Guider J, Scott L. Esophageal Rings and Stricture Related to a Circumferential Inlet Patch. ACG Case Rep J 2016; 3: e124 [PMID: 27807576 DOI: 10.14309/crj.2016.97]
40 Rogart JN, Siddiqui UD. Inlet patch presenting with food impaction caused by peptic stricture. Clin Gastroenterol Hepatol 2007; 5: e35-e36 [PMID: 17683992 DOI: 10.1016/j.cgh.2007.05.025]
41 Yarborough CS, McLane RC. Stricture related to an inlet patch of the esophagus. Am J Gastroenterol 1993; 88: 275-276 [PMID: 8424433]
42 Rodríguez-Martínez A, Salazar-Quero JC, Tutau-Gómez C, Espín-Jaime B, Rubio-Murillo M, Pizarro-Martín A. Heterotopic gastric mucosa of the proximal oesophagus (inlet patch): endoscopic prevalence, histological and clinical characteristics in paediatric patients. Eur J Gastroenterol Hepatol 2014; 26: 1139-1145 [PMID: 25099680 DOI: 10.1097/MEG.0000000000000177]
43 Forcione DG, Lauwers GY, Garber JJ. Eosinophilic gastritis with involvement of esophageal gastric inlet patch. Gastrointest Endosc 2018; 87: 1356-1358 [PMID: 29108982 DOI: 10.1016/j.gie.2017.10.020]
44 Hamada K, Yamasaki Y, Kubota J, Okada H. Gastrointestinal: The first report of an esophageal xanthoma in the cervical inlet patch. J Gastroenterol Hepatol 2018; 33: 1938 [PMID: 30084136 DOI: 10.1111/jgh.14386]
45 Zhou C, Kirtane T, Tsai TH, Lee HC, Adler DC, Schmitt JM, Huang Q, Fujimoto JG, Mashimo H. Cervical inlet patch-optical coherence tomography imaging and clinical significance. World J Gastroenterol 2012; 18: 2502-2510 [PMID: 22654447 DOI: 10.3748/wjg.v18.i20.2502]
46 Kwiatek MA, Mirza F, Kahrilas PJ, Pandolfino JE. Hyperdynamic upper esophageal sphincter pressure: a manometric observation in patients reporting globus sensation. Am J Gastroenterol 2009; 104: 289-298 [PMID: 19174789 DOI: 10.1038/ajg.2008.150]
47 Corso MJ, Pursnani KG, Mohiuddin MA, Gideon RM, Castell JA, Katzka DA, Katz PO, Castell DO. Globus sensation is associated with hypertensive upper esophageal sphincter but not with gastroesophageal reflux. Dig Dis Sci 1998; 43: 1513-1517 [PMID: 9690388 DOI: 10.1023/A:1018862814873]
48 Tokashiki R, Funato N, Suzuki M. Globus sensation and increased upper esophageal sphincter pressure with distal esophageal acid perfusion. Eur Arch Otorhinolaryngol 2010; 267: 737-741 [PMID: 19882344 DOI: 10.1007/s00405-009-1134-1]
49 Rosztóczy A, Izbéki F, Németh IB, Dulic S, Vadászi K, Róka R, Gecse K, Gyökeres T, Lázár G, Tiszlavicz L, Wittmann T. Detailed esophageal function and morphological analysis shows high prevalence of gastroesophageal reflux disease and Barrett’s esophagus in patients with cervical inlet patch. Dis Esophagus 2012; 25: 498-504 [PMID: 22107367 DOI: 10.1111/j.1442-2050.2011.01281.x]
50 Probst A, Schaller T, Messmann H. Adenocarcinoma arising from ectopic gastric mucosa in an esophageal inlet patch: treatment by endoscopic submucosal dissection. Endoscopy 2015; 47 Suppl 1 UCTN: E337-E338 [PMID: 26134434 DOI: 10.1055/s-0034-1392423]
51 Möschler O, Vieth M, Müller MK. Endoscopic resection of an adenocarcinoma occurring in ectopic gastric mucosa within the proximal esophagus. Endoscopy 2014; 46 Suppl 1 UCTN: E24-E25 [PMID: 24523165 DOI: 10.1055/s-0033-1358807]
52 Govani SM, Metko V, Rubenstein JH. Prevalence and risk factors for heterotopic gastric mucosa of the upper esophagus among men undergoing routine screening colonoscopy. Dis Esophagus 2015; 28: 442-447 [PMID: 24758607 DOI: 10.1111/dote.12221]
53 CARRIE A. Adenocarcinoma of the upper end of the oesophagus arising from ectopic gastric epithelium. Br J Surg 1950; 37: 474 [PMID: 15414304 DOI: 10.1002/bjs.18003714810]
54 Yamada T, Tsuji A, Onoue S, Kaneko M, Tanioka F, Osawa S, Saida Y. Acid suppressive therapy improved symptoms due to circumferential cervical inlet patch with proton pumps (H+/K+-ATPase). World J Clin Cases 2017; 5: 403-406 [PMID: 29204429 DOI: 10.12998/wjcc.v5.i11.403]
55 Kawada K, Kawano T, Sugimoto T, Yamaguchi K, Kawamura Y, Matsui T, Okuda M, Ogo T, Kume Y, Nakajima Y, Mora A, Okada T, Hoshino A, Tokairin Y, Nakajima Y, Okada R, Kiyokawa Y, Nomura F, Asakage T, Shimoda R, Ito T. Case of Superficial Cancer Located at the Pharyngoesophageal Junction Which Was Dissected by Endoscopic Laryngopharyngeal Surgery Combined with Endoscopic Submucosal Dissection. Case Rep Otolaryngol 2017; 2017: 1341059 [PMID: 28154766 DOI: 10.1155/2017/1341059]
56 Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc 2004; 59: 288-295 [PMID: 14745410 DOI: 10.1016/S0016-5107(03)02532-X]
57 Inoue H, Kaga M, Ikeda H, Sato C, Sato H, Minami H, Santi EG, Hayee B, Eleftheriadis N. Magnification endoscopy in esophageal squamous cell carcinoma: a review of the intrapapillary capillary loop classification. Ann Gastroenterol 2015; 28: 41-48 [PMID: 25608626]
58 van der Sommen F, Zinger S, Curvers WL, Bisschops R, Pech O, Weusten BL, Bergman JJ, de With PH, Schoon EJ. Computer-aided detection of early neoplastic lesions in Barrett’s esophagus. Endoscopy 2016; 48: 617-624 [PMID: 27100718 DOI: 10.1055/s-0042-105284]
59 Kadota T, Fujii S, Oono Y, Imajoh M, Yano T, Kaneko K. Adenocarcinoma arising from heterotopic gastric mucosa in the cervical esophagus and upper thoracic esophagus: two case reports and literature review. Expert Rev Gastroenterol Hepatol 2016; 10: 405-414 [PMID: 26610162 DOI: 10.1586/17474124.2016.1125780]
60 Abe T, Hosokawa M, Kusumi T, Kusano M, Hokari K, Kagaya H, Watanabe A, Fujita M, Sasaki S. Adenocarcinoma arising from ectopic gastric mucosa in the cervical esophagus. Am J Clin Oncol 2004; 27: 644-645 [PMID: 15577449 DOI: 10.1097/01.coc.0000147808.63442.b5]
61 Alrawi SJ, Winston J, Tan D, Gibbs J, Loree TR, Hicks W, Rigual N, Lorè JM Jr. Primary adenocarcinoma of cervical esophagus. J Exp Clin Cancer Res 2005; 24: 325-330 [PMID: 16110768]
62 Noguchi T, Takeno S, Takahashi Y, Sato T, Uchida Y, Yokoyama S. Primary adenocarcinoma of the cervical esophagus arising from heterotopic gastric mucosa. J Gastroenterol 2001; 36: 704-709 [PMID: 11686482]
63 Georges A, Coopman S, Rebeuh J, Molitor G, Rebouissoux L, Dabadie A, Kalach N, Lachaux A, Michaud L. Inlet patch: clinical presentation and outcome in children. J Pediatr Gastroenterol Nutr 2011; 52: 419-423 [PMID: 21240021 DOI: 10.1097/MPG.0b013e3181f2a913]
64 Akanuma N, Hoshino I, Akutsu Y, Shuto K, Shiratori T, Kono T, Uesato M, Sato A, Isozaki Y, Maruyama T, Takeshita N, Matsubara H. Primary esophageal adenocarcinoma arising from heterotopic gastric mucosa: report of a case. Surg Today 2013; 43: 446-451 [PMID: 22706784 DOI: 10.1007/s00595-012-0206-9]
65 Kim EA, Kang DH, Cho HS, Park DK, Kim YK, Park HC, Kim JH. Acid secretion from a heterotopic gastric mucosa in the upper esophagus demonstrated by dual probe 24-hour ambulatory pH monitoring. Korean J Intern Med 2001; 16: 14-17 [PMID: 11417299 DOI: 10.3904/kjim.2001.16.1.14]
66 Silvers WS, Levine JS, Poole JA, Naar E, Weber RW. Inlet patch of gastric mucosa in upper esophagus causing chronic cough and vocal cord dysfunction. Ann Allergy Asthma Immunol 2006; 96: 112-115 [PMID: 16440542 DOI: 10.1016/S1081-1206(10)61049-6]
67 Hamilton JW, Thune RG, Morrissey JF. Symptomatic ectopic gastric epithelium of the cervical esophagus. Demonstration of acid production with Congo red. Dig Dis Sci 1986; 31: 337-342 [PMID: 3956328]
68 Jeon HK, Kim GH. Can Nocturnal Acid-breakthrough Be Reduced by Long-acting Proton Pump Inhibitors? J Neurogastroenterol Motil 2017; 23: 145-148 [PMID: 28372039 DOI: 10.5056/jnm17037]
69 Peghini PL, Katz PO, Castell DO. Ranitidine controls nocturnal gastric acid breakthrough on omeprazole: a controlled study in normal subjects. Gastroenterology 1998; 115: 1335-1339 [PMID: 9834259]
70 Wang Y, Pan T, Wang Q, Guo Z. Additional bedtime H2-receptor antagonist for the control of nocturnal gastric acid breakthrough. Cochrane Database Syst Rev 2009; : CD004275 [PMID: 19821323 DOI: 10.1002/14651858.CD004275.pub3]
71 Katz PO, Tutuian R. Histamine receptor antagonists, proton pump inhibitors and their combination in the treatment of gastro-oesophageal reflux disease. Best Pract Res Clin Gastroenterol 2001; 15: 371-384 [PMID: 11403533 DOI: 10.1053/bega.2001.0185]
72 Chong VH, Jalihal A. Cervical inlet patch: case series and literature review. South Med J 2006; 99: 865-869 [PMID: 16929882 DOI: 10.1097/01.smj.0000231246.28273.b0]
73 Campbell R, Kilty SJ, Hutton B, Bonaparte JP. The Role of Helicobacter pylori in Laryngopharyngeal Reflux. Otolaryngol Head Neck Surg 2017; 156: 255-262 [PMID: 27803078 DOI: 10.1177/0194599816676052]
74 Dunn JM, Sui G, Anggiansah A, Wong T. Radiofrequency ablation of symptomatic cervical inlet patch using a through-the-scope device: a pilot study. Gastrointest Endosc 2016; 84: 1022-1026.e2 [PMID: 27373671 DOI: 10.1016/j.gie.2016.06.037]
75 McBride MA, Vanagunas AA, Breshnahan JP, Barch DB. Combined endoscopic thermal electrocoagulation with high dose omeprazole therapy in complicated heterotopic gastric mucosa of the esophagus. Am J Gastroenterol 1995; 90: 2029-2031 [PMID: 7485016]
76 Sauvé G, Croué A, Denez B, Boyer J. High-grade dysplasia in heterotopic gastric mucosa in the upper esophagus after radiotherapy: successful eradication 2 years after endoscopic treatment by argon plasma coagulation. Endoscopy 2001; 33: 732 [PMID: 11490394 DOI: 10.1055/s-2001-16221]
77 Pech O, May A, Gossner L, Rabenstein T, Ell C. Management of pre-malignant and malignant lesions by endoscopic resection. Best Pract Res Clin Gastroenterol 2004; 18: 61-76 [PMID: 15123085 DOI: 10.1016/S1521-6918(03)00104-5]
78 Kristo I, Rieder E, Paireder M, Schwameis K, Jomrich G, Dolak W, Parzefall T, Riegler M, Asari R, Schoppmann SF. Radiofrequency ablation in patients with large cervical heterotopic gastric mucosa and globus sensation: Closing the treatment gap. Dig Endosc 2018; 30: 212-218 [PMID: 28884487 DOI: 10.1111/den.12959]
79 Manner H, May A, Miehlke S, Dertinger S, Wigginghaus B, Schimming W, Krämer W, Niemann G, Stolte M, Ell C. Ablation of nonneoplastic Barrett’s mucosa using argon plasma coagulation with concomitant esomeprazole therapy (APBANEX): a prospective multicenter evaluation. Am J Gastroenterol 2006; 101: 1762-1769 [PMID: 16817835 DOI: 10.1111/j.1572-0241.2006.00709.x]
80 Klare P, Meining A, von Delius S, Wolf P, Konukiewitz B, Schmid RM, Bajbouj M. Argon plasma coagulation of gastric inlet patches for the treatment of globus sensation: it is an effective therapy in the long term. Digestion 2013; 88: 165-171 [PMID: 24157960 DOI: 10.1159/000355274]
81 Meining A, Bajbouj M, Preeg M, Reichenberger J, Kassem AM, Huber W, Brockmeyer SJ, Hannig C, Höfler H, Prinz C, Schmid RM. Argon plasma ablation of gastric inlet patches in the cervical esophagus may alleviate globus sensation: a pilot trial. Endoscopy 2006; 38: 566-570 [PMID: 16802267 DOI: 10.1055/s-2006-925362]
82 Qumseya B, Panossian AM, Rizk C, Cangemi D, Wolfsen C, Raimondo M, Woodward T, Wallace MB, Wolfsen H. Predictors of esophageal stricture formation post endoscopic mucosal resection. Clin Endosc 2014; 47: 155-161 [PMID: 24765598 DOI: 10.5946/ce.2014.47.2.155]
83 Kristo I, Asari R, Rieder E, Riegler V, Schoppmann SF. Treatment of Barrett’s esophagus: update on new endoscopic surgical modalities. Minerva Chir 2015; 70: 107-118 [PMID: 25645114]
84 Manner H, May A, Kouti I, Pech O, Vieth M, Ell C. Efficacy and safety of Hybrid-APC for the ablation of Barrett’s esophagus. Surg Endosc 2016; 30: 1364-1370 [PMID: 26104794 DOI: 10.1007/s00464-015-4336-1]
85 Prueksapanich P, Pittayanon R, Rerknimitr R, Wisedopas N, Kullavanijaya P. Value of probe-based confocal laser endomicroscopy (pCLE) and dual focus narrow-band imaging (dNBI) in diagnosing early squamous cell neoplasms in esophageal Lugol’s voiding lesions. Endosc Int Open 2015; 3: E281-E288 [PMID: 26356321 DOI: 10.1055/s-0034-1391903]
P-Reviewer: Sandhu DS
S-Editor: Ma RY L-Editor: E-Editor:
Specialty type: Gastroenterology and hepatology
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
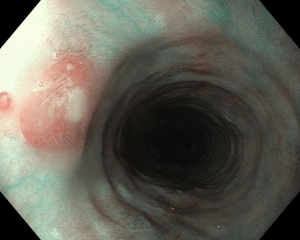
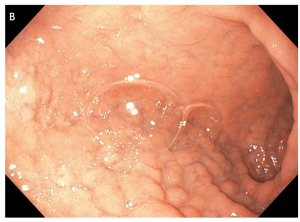
Figure 1 A discreet area of flat salmon pink mucosa with mucus on surface typically found in the proximal esophagus of a 45-year-old anxious woman with globus sensation.
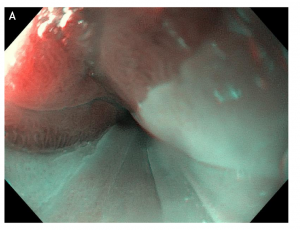
Figure 2 Double mirror flat inlet patches in (A) white light endoscopy vs (B) optical chromoendoscopy (narrow band imaging), in a middle age woman with Helicobacter pylori-associated gastritis and globus sensation ameliorated after the eradication therapy.
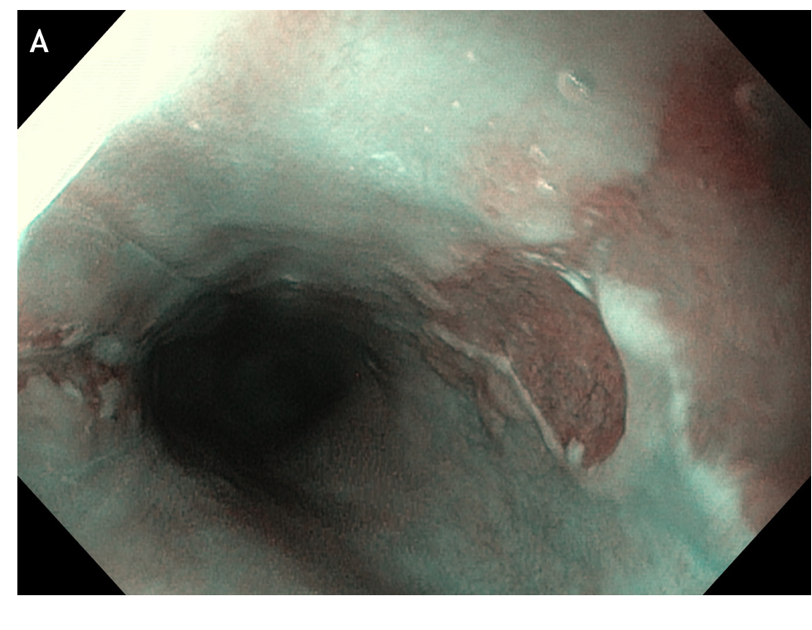
Figure 3 Concomitant findings of inlet patches in patients with inflammatory bowel disease, celiac disease, neurofibromatosis or blue rubber bleb nevus syndrome are likely to be incidental. A: Large inlet patch from 16 to 20 cm from the incisors in a young woman with globus sensation and concomitant celiac disease. The patient underwent an upper endoscopy because of persistent iron deficiency anemia. B: Nodular appearance of duodenal mucosa and C: flattened villi in the same patient.
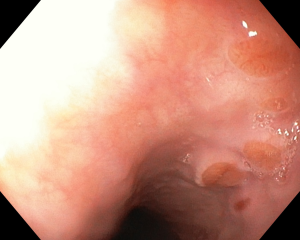
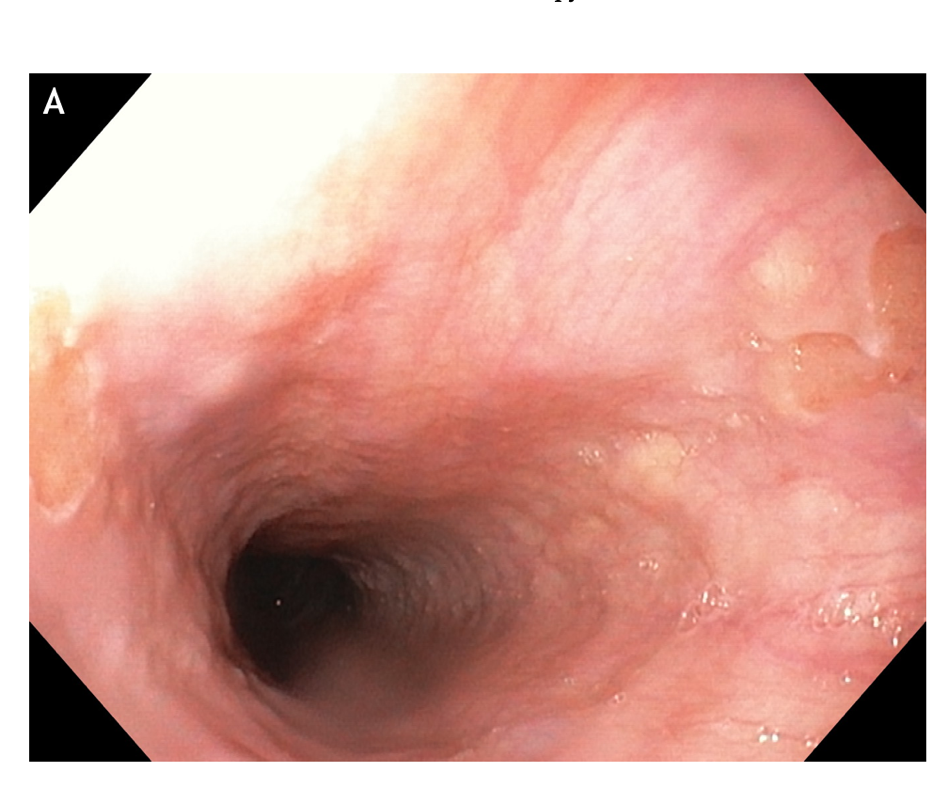
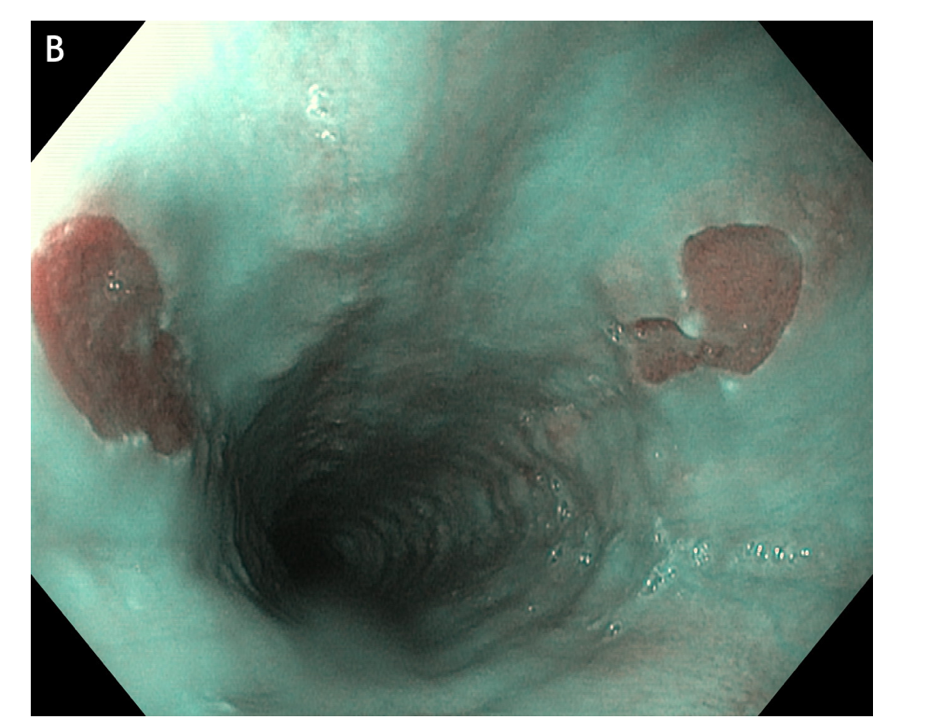
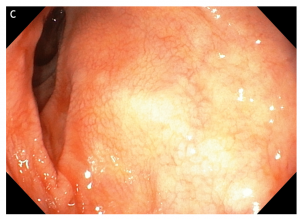
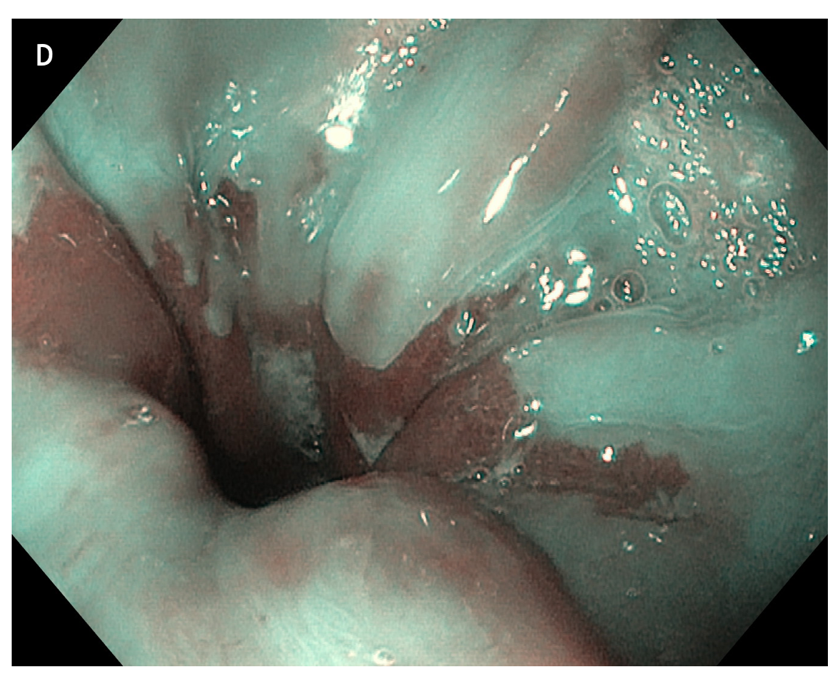
Figure 4 Multiple small focal areas round in shape of gastric tissue, one of them slightly raised, noted in the right lateral field, 10 cm from the incisors, in a young man presenting for unexplained upper dysphagia.
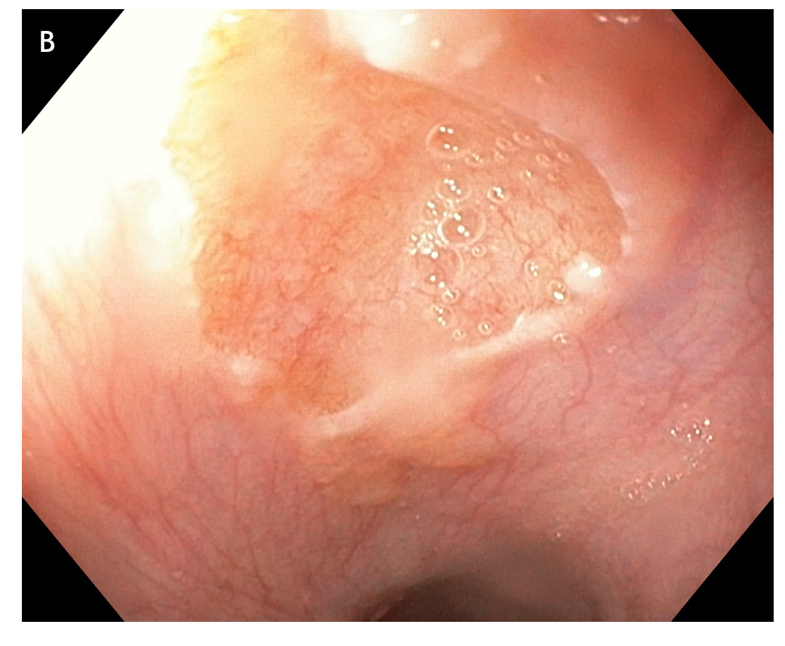
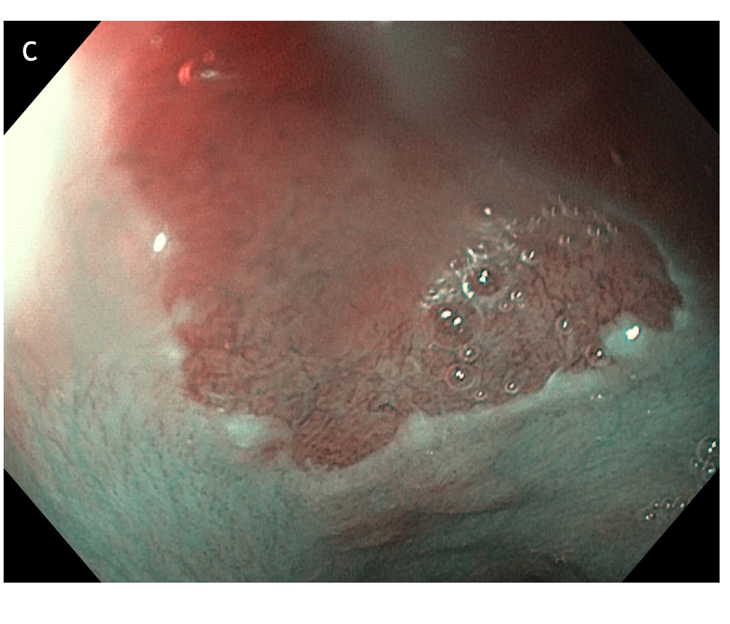
Figure 5 large areas of resected tissue and multiple lesions were independent predictors of stricture formation. A: Three areas of cervical inlet patches, with kissing distribution, in a middle age women with uterine cancer history, presenting for reflux complaints and globus sensation. Detailed image in (B) white light endoscopy and (C) narrow band imaging. D: Irregular Z line in the same patient suggesting concomitant gastroesophageal reflux disease.